- AFP
- 9 Hours ago

AstraZeneca admits potential for blood clots from Covid vaccine in rare instances
-

- Web Desk
- May 01, 2024
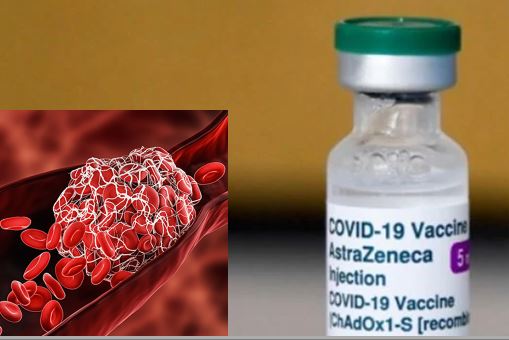
WEB DESK: For the first time, AstraZeneca – the renowned pharmaceutical company known for its contributions during the Covid-19 pandemic – has admitted to the possibility of rare side effects associated with its Covishield vaccine, including blood clots and low platelet count.
The acknowledgment comes amidst a class action lawsuit against the pharma giant, alleging cases of death and severe injury purportedly linked to its vaccine developed in partnership with the University of Oxford.
Health activists sound alarm over potential introduction of 10-stick cigarette packs
According to a news report by The Telegraph, court documents quote the company admission, “It is admitted that the AZ vaccine can, in very rare cases, cause TTS (Thrombosis with Thrombocytopenia Syndrome). The causal mechanism is not known.”
According to the Council for International Organisations of Medical Sciences, “very rare” side effects are those reported in less than 1 in 10,000 cases.
Covishield, a product of collaboration between the British-Swedish company and Oxford University, manufactured by the Serum Institute of India, saw widespread distribution across more than 150 nations.
Despite studies indicating its efficacy ranging between 60 to 80 percent in shielding against the novel coronavirus allegations persist. One plaintiff alleged a permanent brain injury stemming from a vaccine-induced blood clot.
While AstraZeneca disputes these claims, their recent court filing marks the first time they have acknowledged the vaccine’s potential to cause side effects characterised by blood clotting and low platelet count.
Hepatitis B on the verge of becoming deadliest viral disease
According to a news article by The Independent, the company’s stance contradicts its previous assertion in 2023 where it refused to accept a generic link between the vaccine and TTS (Thrombosis with Thrombocytopenia Syndrome).
The emergence of a condition termed vaccine-induced immune thrombocytopenia and thrombosis (VITT) in March 2021 first highlighted a potential association between the vaccine and adverse effects.
Despite the UK government indemnifying AstraZeneca against legal action, it has refrained from intervention.





